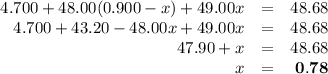Chemistry, 12.09.2019 21:30 SuperWoman9172
Ahypothetical element has an atomic weight of 48.68 amu. it consists of three isotopes having masses of 47.00 amu, 48.00 amu, and 49.00 amu. the lightest-weight isotope has a natural abundance of 10.0%. what is the percent abundance of the heaviest isotope?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 12:50
Use the standard enthalpies of formation for the reactants and products to solve for the δhrxn for the following reaction. (the δhf of c2h4 is 52.26 kj/mol, co2 is -393.509 kj/mol, and h2o is -241.818 kj.) c2h4 (g) + 3o2(g) 2co2 (g) + 2h2o(g) δhrxn = the reaction is .
Answers: 3

Chemistry, 22.06.2019 09:20
Explain that newton first law,second law and third law of motion?
Answers: 2

Chemistry, 22.06.2019 18:10
Areader can tell that the meaning of “obnoxious” will include “having the quality of something” because of the .a) prefix b)pronunciation c)suffix d) word root
Answers: 3

Chemistry, 22.06.2019 18:50
At stp, which substance is the best conductor of electricity? a. nitrogen b. neon c. sulfur d. silver
Answers: 1
You know the right answer?
Ahypothetical element has an atomic weight of 48.68 amu. it consists of three isotopes having masses...
Questions


Mathematics, 13.10.2019 13:00


History, 13.10.2019 13:00

Mathematics, 13.10.2019 13:00



Biology, 13.10.2019 13:00



Mathematics, 13.10.2019 13:00

Physics, 13.10.2019 13:00




Physics, 13.10.2019 13:00

Mathematics, 13.10.2019 13:00

History, 13.10.2019 13:00


Biology, 13.10.2019 13:00