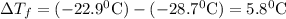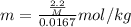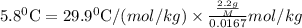Asolution was prepared by dissolving 2.2 g of an unknown solute in 16.7 g of ccl4. a thermal analysis was performed for this solution and it was found that its initial freezing point was – 28.7°c. a reliable source in the bibliography states that for ccl4, t°f = – 22.9°c, and its freezing point lowering constant is kf = 29.9°c/m. calculate the molar mass of the unknown solute.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 19:00
In any energy conversion, some of the energy is lost to the environment as question 5 options: electrical energy potential energy sound energy thermal energy
Answers: 1

Chemistry, 22.06.2019 06:30
What is the correct term for living the most sustainable life you can within your current circumstances?
Answers: 1

Chemistry, 22.06.2019 11:00
Surface currents are caused by blank space . question 14 options: surface currents are caused by? differences in water temperature high salinity differences in density wind forces
Answers: 1

You know the right answer?
Asolution was prepared by dissolving 2.2 g of an unknown solute in 16.7 g of ccl4. a thermal analysi...
Questions


Mathematics, 17.11.2019 18:31




Business, 17.11.2019 18:31

Mathematics, 17.11.2019 18:31


Chemistry, 17.11.2019 18:31

Mathematics, 17.11.2019 18:31



Chemistry, 17.11.2019 18:31



Biology, 17.11.2019 18:31

English, 17.11.2019 18:31

Chemistry, 17.11.2019 18:31


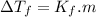
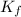 is cryogenoscopic constant of solvent.
is cryogenoscopic constant of solvent.