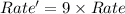Consider the following reaction: 2 no(g) + 2h2(g) → n2(g) + 2 h2o(g) the rate law for this reaction is first order in h2 and second order in no. what would happen to the rate if the initial concentration of no tripled while all other factors stayed the same? the rate will increase by a factor of 9. the rate will decrease by a factor of 3. the rate will double. the rate will triple. the rate will remain constant.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:30
Liv sheldon given the balanced equation for an organic reaction: c2h2 + 2cl2 → c2h2cl4 this reaction is best classified as *
Answers: 1

Chemistry, 22.06.2019 06:00
An alkaline battery produces electrical energy according to the following equation. zn(s) + 2 mno2(s) + h2o(l) zn(oh)2(s) + mn2o3(s) (a) determine the limiting reactant if 17.5 g zn and 31.0 g mno2 are used. (type your answer using the format ch4 for ch4.) (b) determine the mass of zn(oh)2 produced. _ g
Answers: 3

Chemistry, 22.06.2019 09:30
Melissa is interested in her family tree and how her family has changed over its many generations. melissa probably more closely resembles
Answers: 2

Chemistry, 23.06.2019 00:30
When did stem cell research become known ? who discovered stem cell? what experiments or studies have been conducted so far?
Answers: 3
You know the right answer?
Consider the following reaction: 2 no(g) + 2h2(g) → n2(g) + 2 h2o(g) the rate law for this reaction...
Questions


Mathematics, 06.01.2021 21:40





Chemistry, 06.01.2021 21:40

Mathematics, 06.01.2021 21:40

Geography, 06.01.2021 21:40

Mathematics, 06.01.2021 21:40



Mathematics, 06.01.2021 21:40

Mathematics, 06.01.2021 21:40


Physics, 06.01.2021 21:40

World Languages, 06.01.2021 21:40



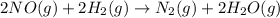
 = 2
= 2 = 1
= 1![Rate=k[NO]^2[H_2]^1](/tpl/images/0229/1480/39530.png)
![Rate'=k[3\times NO]^2[H_2]^1](/tpl/images/0229/1480/e80a2.png)
![Rate'=k[3]^2[NO]^2[H_2]^1](/tpl/images/0229/1480/b88df.png)
![Rate'=k\times 9[NO]^2[H_2]^1](/tpl/images/0229/1480/d2dcb.png)