
Chemistry, 12.09.2019 20:30 Pointjazzyqueen602
An aerosol can with a temperature of 292 k and a pressure of 1.25 atm was left outside in the sun on a hot summer day. the pressure inside the can rose to 1.33 atm. what was the resulting temperature in the can?
322k
1,600k
311k
274k
ik its not a

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 23:00
What is the most common reason for matter changing its state?
Answers: 1

Chemistry, 22.06.2019 23:30
Imagine a small synthetic vesicle made from pure phospholipids enclosing an interior lumen containing 1 mm glucose and 1 mm sodium chloride. if the vesicle is placed in pure water, which of the following happens faster? a. na+ diffuses out. b. cl– diffuses out. c. h2o diffuses in. d. glucose diffuses out. e. sodium chloride diffuses out.
Answers: 3

Chemistry, 23.06.2019 03:20
High-pressure liquid chromatography (hplc) is a method used in chemistry and biochemistry to purify chemical substances. the pressures used in this procedure range from around 500 kilopascals (500,000 pa) to about 60,000 kpa (60,000,000 pa). it is often convenient to know the pressure in torr. if an hplc procedure is running at a pressure of 1.03×108 pa , what is its running pressure in torr?
Answers: 3

Chemistry, 23.06.2019 11:00
Afraction can be converted to a decimal by dividing the denominator into the numerator. use a calculator. divide to convert the fractions from part a to decimals. then describe the pattern you see in the decimal.
Answers: 3
You know the right answer?
An aerosol can with a temperature of 292 k and a pressure of 1.25 atm was left outside in the sun on...
Questions


Spanish, 01.04.2020 21:35

Mathematics, 01.04.2020 21:35


Mathematics, 01.04.2020 21:35



Mathematics, 01.04.2020 21:35

Mathematics, 01.04.2020 21:35


Mathematics, 01.04.2020 21:35

English, 01.04.2020 21:35


Mathematics, 01.04.2020 21:35

Mathematics, 01.04.2020 21:35





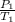 =
= 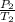
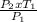
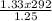 = 310.69K
= 310.69K

