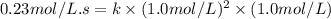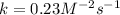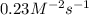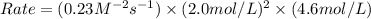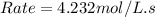Chemistry, 12.09.2019 20:20 juliaduenkelsbu
Enter your answer in the provided box. remember to enter your answer to the correct number of significant figures. for the reaction: a(g) + b(g) → ab(g) the rate is 0.23 mol/l·s, when [a]0 = [b]0 = 1.0 mol/l. if the reaction is first order in b and second order in a, what is the rate when [a]0 = 2.0 mol/l and [b]0 = 4.6 mol/l?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 16:50
What is the composition, in atom percent, of an alloy that consists of 4.5 wt% pb and 95.5 wt% sn? the atomic weights for pb and sn are 207.19 g/mol and 118.71 g/mol, respectively.(a) 2.6 at% pb and 97.4 at% sn(b) 7.6 at% pb and 92.4 at% sn(c)97.4 at% pb and 2.6 at% sn(d) 92.4 at% pb and 7.6 at% sn
Answers: 2

Chemistry, 22.06.2019 20:30
How many grams of phosphorus are contained in 5.09 moles of phosphorus?
Answers: 1

Chemistry, 22.06.2019 22:30
What is a number added in front of a formula in order to balance the equation
Answers: 1

Chemistry, 23.06.2019 01:10
A5.00 g of a in . g of at aa 5.00 g of b in . g of .?at .
Answers: 1
You know the right answer?
Enter your answer in the provided box. remember to enter your answer to the correct number of signif...
Questions

English, 06.05.2020 12:58



Mathematics, 06.05.2020 12:58

Computers and Technology, 06.05.2020 12:58


English, 06.05.2020 12:58


History, 06.05.2020 12:58

Mathematics, 06.05.2020 12:58


Chemistry, 06.05.2020 12:58

Social Studies, 06.05.2020 12:58

Physics, 06.05.2020 12:58


History, 06.05.2020 12:58

Mathematics, 06.05.2020 12:58


Mathematics, 06.05.2020 12:58

Mathematics, 06.05.2020 12:58

![Rate=k[A]^2[B]](/tpl/images/0229/0825/4c585.png)