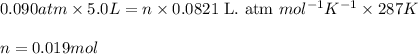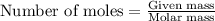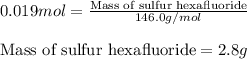Sulfur hexafluoride gas is collected at 14.0°c in an evacuated flask with a measured volume of 5.0l. when all the gas has been collected, the pressure in the flask is measured to be 0.090atm . calculate the mass and number of moles of sulfur hexafluoride gas that were collected. be sure your answer has the correct number of significant digits.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:20
Why does the sun appear to be the brightest star in the sky? a- its apparent brightness is much greater than other stars. b- it burns more gas, making it brighter than any other star. c- it is the largest star in the galaxy, so it is the brightest star. d- its relative distance to earth is closer than the other stars.
Answers: 1

Chemistry, 22.06.2019 13:00
Jose and eric were given four samples in lab. the results of their analysis are shown in the table. based on the data they collected, which sample is most likely a metal?
Answers: 1

Chemistry, 22.06.2019 15:00
According to the diagram, what sources contribute to the phosphorus found in soil? according to the diagram, phosphorus found in soil contributes phosphorus to what other sources?
Answers: 1

You know the right answer?
Sulfur hexafluoride gas is collected at 14.0°c in an evacuated flask with a measured volume of 5.0l....
Questions


History, 20.04.2020 19:49



Chemistry, 20.04.2020 19:49


Mathematics, 20.04.2020 19:49


Mathematics, 20.04.2020 19:49


Mathematics, 20.04.2020 19:49

History, 20.04.2020 19:49


History, 20.04.2020 19:49





Chemistry, 20.04.2020 19:49

Mathematics, 20.04.2020 19:49

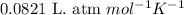
![14^oC=[273+14]K=287K](/tpl/images/0229/0798/930e3.png)