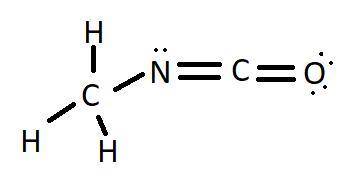Methyl isocyanate, h3c-n=c=o, is used in the industrial synthesis of a type of pesticide and herbicide known as a carbamate. as a historical note, an industrial accident in bhopal, india in 1984 resulted in leakage of an unknown quantity of this chemical into the air. an estimated 200,000 persons were exposed to its vapors and over 2000 of these people died.
(a) draw the lewis structure for methyl isocyanate.
explicitly draw all h atoms.
include all valence lone pairs in your answer.
(b) what is the hybridization of the carbonyl carbon? ²spsp³
what is the hybridization of the nitrogen? ²spsp³

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:30
Which of the following two events occur to create a sea breeze? select all that apply. warm air rises on the ocean and moves toward the land to cool warm air rises on land and moves toward the ocean to cool cool air moves from the ocean to be warmed by the land cool air moves from the land to be warmed by the ocean
Answers: 3

Chemistry, 22.06.2019 08:50
If two atoms are bonded to a central atom with no lone pairs,how will they be arranged
Answers: 3

Chemistry, 22.06.2019 12:00
Hey guys so i need to know what is _nh3+> nh4oh ~chemistry~
Answers: 1

Chemistry, 22.06.2019 16:00
Click the button that shows the correct relationship of the electron affinities of the elements sodium and phosphorus. sodium’s electron affinity value is more negative than the electron affinity value of phosphorus. phosphorus’ electron affinity value is more negative than the electron affinity value of sodium. this information cannot be determined using the periodic table. answer is b on e2020.
Answers: 3
You know the right answer?
Methyl isocyanate, h3c-n=c=o, is used in the industrial synthesis of a type of pesticide and herbici...
Questions


English, 05.05.2020 04:35


English, 05.05.2020 04:35

Mathematics, 05.05.2020 04:35

English, 05.05.2020 04:35


Mathematics, 05.05.2020 04:36


Mathematics, 05.05.2020 04:36

Mathematics, 05.05.2020 04:36


Mathematics, 05.05.2020 04:36


Mathematics, 05.05.2020 04:36




Biology, 05.05.2020 04:36