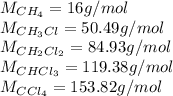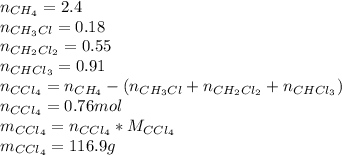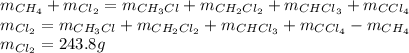Chemistry, 12.09.2019 19:20 tishfaco5000
Methane and chlorine react to form four products: ch3cl, ch2cl2, chcl3, and ccl4. at a particular temperature and pressure, 38.4 g of ch4 was allowed to react with excess cl2 and gave 9.2 g ch3cl, 47.1 g ch2cl2, and 109 g chcl3. all the ch4 reacted. (note: the hydrogen that is displaced from the carbon also combines with cl2 to form hcl.)how many grams of ccl4 were formed? how many grams of cl2 reacted with the ch4?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:30
In pea plants, the allele for tallness (t) is dominant to the allele for shortness (t). in the cross between a tall pea plant and a short pea plant shown below, what is the probability that the resulting offspring will be tall? whats the percent
Answers: 1

Chemistry, 22.06.2019 15:40
Use the periodic table to complete this equation that represents nuclear fission processesun - ba c 3 n
Answers: 2

Chemistry, 22.06.2019 16:30
Explain in detail of the four major scientific developments that spurred the formulation of the plate tectonics theory
Answers: 2

Chemistry, 22.06.2019 17:30
Energy defines the different "states" of matter. in no more than 3 sentences, describe the amount of kinetic energy that each of the 3 states of matter possesses and relate that to the atom/molecular motion of each "state".
Answers: 2
You know the right answer?
Methane and chlorine react to form four products: ch3cl, ch2cl2, chcl3, and ccl4. at a particular t...
Questions



Social Studies, 14.05.2021 18:00

Biology, 14.05.2021 18:00

Mathematics, 14.05.2021 18:00

Social Studies, 14.05.2021 18:00



Physics, 14.05.2021 18:00

Mathematics, 14.05.2021 18:00


History, 14.05.2021 18:00


Business, 14.05.2021 18:00

Mathematics, 14.05.2021 18:00

Chemistry, 14.05.2021 18:00


Business, 14.05.2021 18:00

History, 14.05.2021 18:00

Mathematics, 14.05.2021 18:00