
Chromium has an atomic mass of 51.9961 u and consists of four isotopes, cr50, cr52, cr53, and cr54. the cr52 isotope has a natural abundance of 83.79% and an atomic mass of 51.9405 u. the cr54 isotope has a natural abundance of 2.37% and an atomic mass of 53.9389 u. the natural abundances of the cr50 and cr53 isotopes exist in a ratio of 1: 0.4579, and the cr50 isotope has an atomic mass of 49.9460 u. determine the atomic mass of the cr53 isotope.

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 06:10
56.16 gregor mendel was the first scientist to use statistics to analyze scientific data. before mendel's experiments, scientists believed that organisms acquired traits from their environment and passed them on to their offspring. after mendel's discoveries were accepted, scientists realized that traits passed to offspring were the result of genes being passed from parents to offspring. this is an example of pls
Answers: 1

Chemistry, 22.06.2019 08:40
Write the formula for the following chemicals. 7. e. trinitrogen tetraoxide a calcium phosphate f. magnesium acetate b. potassium sulfide g nickel(iii) cyanide c carbon dioxide h. silver sulfate d. cobalt(ii) chloride
Answers: 1

Chemistry, 22.06.2019 12:30
Which of the following describes a compound? (hint: carbon and oxygen bo a. a piece of pure carbon, containing only carbon atoms b. oxygen gas surrounding a solid piece of carbon c. a substance made of two oxygen atoms for each carbon atom carbon and oxygen atoms mixed without being bonded together
Answers: 1
You know the right answer?
Chromium has an atomic mass of 51.9961 u and consists of four isotopes, cr50, cr52, cr53, and cr54....
Questions


Mathematics, 05.06.2020 17:01


Social Studies, 05.06.2020 17:57


Mathematics, 05.06.2020 17:57

Mathematics, 05.06.2020 17:57

Mathematics, 05.06.2020 17:57


Mathematics, 05.06.2020 17:57


Chemistry, 05.06.2020 17:57



Computers and Technology, 05.06.2020 17:57


Mathematics, 05.06.2020 17:57


Mathematics, 05.06.2020 17:57

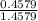 x 13.84 = 4.3469%
x 13.84 = 4.3469%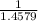 x 13.84u = 9.4931%
x 13.84u = 9.4931%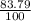 ) + ( 49.9460 x
) + ( 49.9460 x 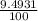 + (m₅₃ x
+ (m₅₃ x 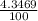 ) + (53.9389 x
) + (53.9389 x 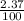 )
)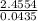 = 56.4459u
= 56.4459u

