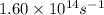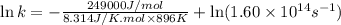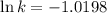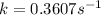Chemistry, 11.09.2019 04:30 shawn20034
Ethyl chloride vapor decomposes by the first-order reaction c2h5cl → c2h4 + hcl the activation energy is 249 kj/mol and the frequency factor is 1.60 × 1014 s−1. find the value of the specific rate constant at 896 k . enter your answer numerically (to 4 decimal places) and in terms of the appropriate units for a first order reaction.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Omg imgonnafailnfiedkla use complete sentences to explain how the mass of hydrogen is conserved during cellular respiration.
Answers: 1

Chemistry, 22.06.2019 05:30
What is the mass defect of a mole of nuclei with 1.8 x 10^15 j/mol binding energy?
Answers: 1

Chemistry, 22.06.2019 16:10
Given the following equation: 2a1 + 3mgcl2 --> 2alcl3 + 3mg how many moles of aluminum chloride are produced from 2.5 moles of magnesium chloride?
Answers: 1

Chemistry, 22.06.2019 19:30
Acetylene gas c2h2 undergoes combustion to produce carbon dioxide and water vapor how many grams of water are produced by the same amount of c2h2?
Answers: 2
You know the right answer?
Ethyl chloride vapor decomposes by the first-order reaction c2h5cl → c2h4 + hcl the activation energ...
Questions

Mathematics, 24.02.2021 20:00


Mathematics, 24.02.2021 20:00

English, 24.02.2021 20:00


Mathematics, 24.02.2021 20:00

Mathematics, 24.02.2021 20:00


Mathematics, 24.02.2021 20:00


Mathematics, 24.02.2021 20:00

Mathematics, 24.02.2021 20:00


Mathematics, 24.02.2021 20:00


Mathematics, 24.02.2021 20:00


Mathematics, 24.02.2021 20:00


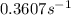
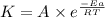
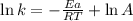 ............(1)
............(1)