
Chemistry, 11.09.2019 03:10 cmflores3245
Asample of pure water is heated to a temperature of 112 c at a pressure of 20 mpa, where the ionization constant for water is 4.0 x 1012. what is the oh concentration in the pure water at these conditions?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 09:20
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2

Chemistry, 22.06.2019 12:00
In the following redox reaction which is the oxidizing agent and which is the reducing agent? alcl3 + na nacl + al oxidizing agent = reducing agent =
Answers: 1

Chemistry, 22.06.2019 17:00
The atoms of a solid aluminum can are close together, vibrating in a rigid structure. if the can is warmed up on a hot plate, what happens to the atoms?
Answers: 1

Chemistry, 23.06.2019 00:00
#7 how does the structure of amino acids allow them to form a polypeptide? each amino acid has an amino group and a carboxyl group. each amino acid has a hydrogen atom and a carboxyl group. each amino acid has a carboxyl group and an r group. each amino acid has an r group and a hydrogen atom.
Answers: 1
You know the right answer?
Asample of pure water is heated to a temperature of 112 c at a pressure of 20 mpa, where the ionizat...
Questions




















![[OH^-]^2=2.0\times 10^{6}](/tpl/images/0227/4886/54276.png)
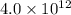 at a temperature of
at a temperature of 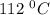 and pressure of 20 MPa
and pressure of 20 MPa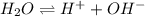
![K_w=[H^+][OH^-]](/tpl/images/0227/4886/bc68a.png)
![[H^+]=[OH^-]](/tpl/images/0227/4886/0669d.png)
![K_w=[OH^-]^2](/tpl/images/0227/4886/d08d4.png)
![[OH^-]^2=4.0\times 10^{12}](/tpl/images/0227/4886/f96fa.png)


