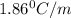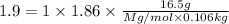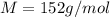Chemistry, 10.09.2019 23:10 blakestuhan
An aqueous solution containing 16.5 g of an unknown molecular (nonelectrolyte) compound in 106.0 g of water was found to have a freezing point of -1.9 ∘c. calculate the molar mass of the unknown compound.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 21:00
Write the chemical symbols for three different atoms or atomic cations with 27 electrons. asap!
Answers: 2

Chemistry, 22.06.2019 04:00
You encounter a solution that is acidic and you decide to test it by adding a small amount of a strong acid. the ph lowers slightly but is approximately unchanged, and still remains acidic. what can you say about the solution? a. it is a buffer solution. b. it is not a buffer solution it is a strong acid solution. d. the solution has been neutralized. e. the solution has excess acid present
Answers: 1


Chemistry, 22.06.2019 18:50
Which of the following is a conclusion that resulted from ernest rutherford’s scattering experiment? (will mark brainliest) a. the nucleus is negatively charged b. the atom is a dense solid and is indivisible c. the mass is conserved when atoms react chemically d. the nucleus is very small and the atom is mostly empty space
Answers: 3
You know the right answer?
An aqueous solution containing 16.5 g of an unknown molecular (nonelectrolyte) compound in 106.0 g o...
Questions

Advanced Placement (AP), 20.09.2021 06:20


Chemistry, 20.09.2021 06:20

Business, 20.09.2021 06:20

Mathematics, 20.09.2021 06:20




Biology, 20.09.2021 06:20

English, 20.09.2021 06:20

Mathematics, 20.09.2021 06:20

Mathematics, 20.09.2021 06:20

Mathematics, 20.09.2021 06:30




Mathematics, 20.09.2021 06:30



Mathematics, 20.09.2021 06:30

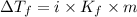
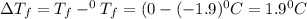 = Depression in freezing point
= Depression in freezing point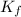 = freezing point constant =
= freezing point constant =