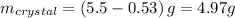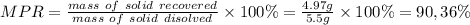Chemistry, 10.09.2019 21:30 Terrydactly
The solubility of acetanilide is 0.53 g in 100 ml of ice‑cold water, and 5.50 g in 100 ml of boiling water. what is the maximum percent recovery that can be achieved for the recrystallization of acetanilide from water?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:30
The speed of light is around 6.706×10^8 miles per hour. what is the speed of light in units of miles per minute?
Answers: 2


Chemistry, 22.06.2019 22:30
Vi limitens. vastery test select the correct answer. which statement explains why large atoms are more reactive than small atoms? a. large atoms have valence electrons farther from the nucleus and lose them more readily. b. large atoms have greater ionization energy, which they can utilize during a reaction. c. large atoms have a greater number of electrons that they can lose during a reaction. d. large atoms have more energy levels, so they have more energy to pass on in a reaction. reset next
Answers: 3

Chemistry, 23.06.2019 06:40
The combustion of methane, ch4, releases 890.4kj/mol. that is, when one mole of methane is burned,890.4 kj are given off to the surroundings. this meansthat the products have 890.4 kj less than the reactants.thus, ah for the reaction = - 890.4 kj. a negative symbolforah indicates an exothermic reaction.ch (g) + 20 (g)> co2 (g) + 2 h0 (1); ah = - 890.4 kga) how much energy is given off when 2.00 mol of ch,are burned? b) how much energy is released when 22.4g of ch. areburned?
Answers: 1
You know the right answer?
The solubility of acetanilide is 0.53 g in 100 ml of ice‑cold water, and 5.50 g in 100 ml of boiling...
Questions

English, 24.04.2021 21:00

Mathematics, 24.04.2021 21:00





History, 24.04.2021 21:10



Mathematics, 24.04.2021 21:10


Health, 24.04.2021 21:10





History, 24.04.2021 21:10



Mathematics, 24.04.2021 21:10