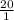Chemistry, 10.09.2019 20:30 dreyiamacc9695
The ph of blood depends on the [hco3-/h2co3] balance. ([h2co3] is equal to the amount of dissolved co2). calculate the bicarbonate (hco3-) : carbon dioxide ratio for a normal blood ph of 7.40. (the pka1 of carbonic acid is 6.10 at 37oc, body temperature). (a) 20 : 1 (b) 1.3 : 1 (c) 2 : 1 (d) 1 : 20 (e) 1 : 0.01

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 21:00
Which of these is an example of pseudoscience? a) predicting the time of sunrise based on data on position of earth b) predicting the date of the moon phases based on data on position of earth c) predicting eclipses based on the position of the sun and the moon d) predicting future events in a person's life based on the position of the moon
Answers: 1

Chemistry, 23.06.2019 20:00
Within nonmetals,ions that have more electrons tend to be bigger than ions that have fewer additional electrons why?
Answers: 1

Chemistry, 23.06.2019 20:40
Who discovered that a dense positively charged atomic nucleus is at the core of every atom
Answers: 1

Chemistry, 24.06.2019 01:30
What is the balanced equation of this chemical reaction ?
Answers: 3
You know the right answer?
The ph of blood depends on the [hco3-/h2co3] balance. ([h2co3] is equal to the amount of dissolved c...
Questions

History, 16.09.2019 07:30

Mathematics, 16.09.2019 07:30


History, 16.09.2019 07:30

Mathematics, 16.09.2019 07:30

Biology, 16.09.2019 07:30

Biology, 16.09.2019 07:30

Mathematics, 16.09.2019 07:30

History, 16.09.2019 07:30

Biology, 16.09.2019 07:30

Chemistry, 16.09.2019 07:30

History, 16.09.2019 07:30

Mathematics, 16.09.2019 07:30

Mathematics, 16.09.2019 07:30

Business, 16.09.2019 07:30

History, 16.09.2019 07:30

Mathematics, 16.09.2019 07:30

Mathematics, 16.09.2019 07:30

Mathematics, 16.09.2019 07:30

![[H_{2}CO_{3}]](/tpl/images/0226/9901/77e8d.png) =
= ![[CO_{2}]](/tpl/images/0226/9901/0a7e9.png)
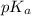 = 6.10
= 6.10![\frac{[HCO_^{-}{3}]}{[CO_{2}]}](/tpl/images/0226/9901/0b823.png) = ?
= ?![pK_{a} + log_{10} \frac{[Salt]}{[Acid]}](/tpl/images/0226/9901/394fa.png)
![pK_{a} + log_{10} \frac{[HCO^{-}_{3}]}{[H_{2}CO_{3}]}](/tpl/images/0226/9901/d9b18.png)
![log_{10} \frac{[HCO^{-}_{3}]}{[H_{2}CO_{3}]}](/tpl/images/0226/9901/e29e6.png)
![\frac{[HCO^{-}_{3}]}{[H_{2}CO_{3}]}](/tpl/images/0226/9901/4d8f1.png) = antilog (1.30)
= antilog (1.30) ![\frac{[HCO^{-}_{3}]}{[CO_{2}]}](/tpl/images/0226/9901/7a396.png) =
=