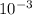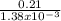The partial pressure of o2 in air at sea level is 0.21atm. the solubility of o2 in water at 20∘c, with 1 atm o2 pressure is 1.38×10−3 m. part a using henry's law, calculate the molar concentration of o2 in the surface water of a mountain lake saturated with air at 20 ∘c and an atmospheric pressure of 665 torr . express your answer using two significant figures. nothing

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 17:30
A650 ml sodium bromine solution has a bromide ion concentration of 0.245 m. what is the mass (g) of sodium bromide in solution? a) 103.b)0.00155.c)16400.d) 16.4.e) 0.159
Answers: 2

Chemistry, 22.06.2019 20:00
Listenbase your answer to the question on the information below.nuclear radiation is harmful to living cells, particularly to fast-growing cells, such as cancer cells and blood cells. an external beam of the radiation emitted from a radioisotope can be directed on a small area of a person to destroy cancer cells within the body.cobalt-60 is an artificially produced radioisotope that emits gamma rays and beta particles. one hospital keeps a 100.0-gram sample of cobalt-60 in an appropriate, secure storage container for future cancer treatment.which choice represents the correct product for the beta decay of the co-60? fe-60ni-60fe-61ni-61
Answers: 2

Chemistry, 23.06.2019 01:00
Which polymers are most closely related? a. protein and nucleic acids b. cellulose and starch c. nucleic acids and starch d. nucleic acids and cellulose
Answers: 2

Chemistry, 23.06.2019 06:30
Which of these natural resources is non-renewable a.corn b.wind c.geothermal d.natural gas
Answers: 2
You know the right answer?
The partial pressure of o2 in air at sea level is 0.21atm. the solubility of o2 in water at 20∘c, wi...
Questions


History, 02.10.2020 16:01


History, 02.10.2020 16:01

Biology, 02.10.2020 16:01


History, 02.10.2020 16:01

History, 02.10.2020 16:01

History, 02.10.2020 16:01

Mathematics, 02.10.2020 16:01

English, 02.10.2020 16:01

Social Studies, 02.10.2020 16:01



Spanish, 02.10.2020 16:01

Health, 02.10.2020 16:01

Mathematics, 02.10.2020 16:01



 M
M