
Chemistry, 10.09.2019 18:30 kevinleon695
Which of the following is not a postulate of the kinetic molecular theory? select one: a. gas particles have most of their mass concentrated in the nucleus of the atom. b. the moving particles undergo perfectly elastic collisions with the walls of the container. c. the forces of attraction and repulsion between the particles are insignificant. d. the average kinetic energy of the particles is directly proportional to the absolute temperature. e. all of the above are postulates of the kinetic molecular theory.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 07:30
Using data from seismic waves, geologists have learned that earth’s interior is made up of several
Answers: 3


Chemistry, 23.06.2019 02:00
The bohr model of the atom explained why emission spectra are discrete. it could also be used to explain the photoelectric effect. which is a correct explanation of the photoelectric effect according to the model?
Answers: 3

Chemistry, 23.06.2019 02:00
What are fossils of organisms that existed over a wide area but only for a limited time period called?
Answers: 2
You know the right answer?
Which of the following is not a postulate of the kinetic molecular theory? select one: a. gas part...
Questions



Physics, 23.05.2021 09:00


Biology, 23.05.2021 09:00

Mathematics, 23.05.2021 09:00



Mathematics, 23.05.2021 09:00

Chemistry, 23.05.2021 09:00

English, 23.05.2021 09:00


Biology, 23.05.2021 09:00


Mathematics, 23.05.2021 09:00

Mathematics, 23.05.2021 09:00

English, 23.05.2021 09:00

Mathematics, 23.05.2021 09:00

Mathematics, 23.05.2021 09:00

Mathematics, 23.05.2021 09:00

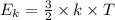 (where T is the absolute temperature and k the Boltzmann constant. This means that kinetic energy is directly proportional to temperature).
(where T is the absolute temperature and k the Boltzmann constant. This means that kinetic energy is directly proportional to temperature).


