
Chemistry, 10.09.2019 18:10 ariellake8551
When m2s3(s) is heated in air, it is converted to mo2(s). a 3.280 g sample of m2s3(s) shows a decrease in mass of 0.228 g when it is heated in air. what is the average atomic mass of m?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 13:50
Amap that uses a range of colors and shading to represent the elevation, depth, or landscape of specific features on earth is a/an map.
Answers: 3

Chemistry, 22.06.2019 15:00
Many ionic compounds and a few highly polar covalent compounds are because they completely ionize in water to create a solution filled with charged ions that can conduct an electric current.
Answers: 1

Chemistry, 23.06.2019 00:50
50 points! need answer asap. what type of organic compound contains the following functional group? (2 points)
Answers: 3

Chemistry, 23.06.2019 01:30
At a certain temperature the rate of this reaction is first order in hi with a rate constant of : 0.0632s2hig=h2g+i2g suppose a vessel contains hi at a concentration of 1.28m . calculate how long it takes for the concentration of hi to decrease to 17.0% of its initial value. you may assume no other reaction is important. round your answer to 2 significant digits.
Answers: 1
You know the right answer?
When m2s3(s) is heated in air, it is converted to mo2(s). a 3.280 g sample of m2s3(s) shows a decrea...
Questions


Engineering, 07.08.2019 19:20










Engineering, 07.08.2019 19:20








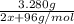
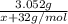
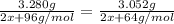
 }" alt="1.075*(2x+64g/mol)=2x+96g/mol\\2.150x+68.8g/mol=2x+96g/mol\\0.150x=27.2g/mol\\x=181.33g/mol" />}" />
}" alt="1.075*(2x+64g/mol)=2x+96g/mol\\2.150x+68.8g/mol=2x+96g/mol\\0.150x=27.2g/mol\\x=181.33g/mol" />}" />

