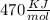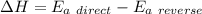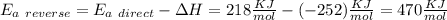The activation energy for the reaction no2 (g )+ co (g) ⟶ no (g) + co2 (g) is ea = 218 kj/mol and the change in enthalpy for the reaction is δh = -252 kj/mol . what is the activation energy for the reverse reaction? enter your answer numerically and in terms of kj/mol.

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 10:40
Which buffer would be better able to hold a steady ph on the addition of strong acid, buffer 1 or buffer 2? explain. buffer 1: a solution containing 0.10 m nh4cl and 1 m nh3. buffer 2: a solution containing 1 m nh4cl and 0.10 m nh3
Answers: 1

Chemistry, 22.06.2019 19:00
Which is the solubility product expression for caf2(s)?  [ca2+]/[f–]2  [ca2+][f2–]  [ca]+[f]2  [ca2+][f–]2
Answers: 3

Chemistry, 23.06.2019 00:00
(04.05 hc) analyze the given diagram of the carbon cycle below. part 1: which compound does c represent? part 2: name a process that could release this compound into the air. part 3: explain how the elements that form it are conserved during the carbon cycle. use complete sentences to explain your answer. justify how this compound was created from a recycling of carbon in the carbon cycle. use complete sentences to explain your answer.
Answers: 3
You know the right answer?
The activation energy for the reaction no2 (g )+ co (g) ⟶ no (g) + co2 (g) is ea = 218 kj/mol and th...
Questions





Mathematics, 02.09.2019 10:30

Physics, 02.09.2019 10:30

Mathematics, 02.09.2019 10:30


Social Studies, 02.09.2019 10:30



Social Studies, 02.09.2019 10:30




Biology, 02.09.2019 10:30


Arts, 02.09.2019 10:30

Chemistry, 02.09.2019 10:30