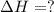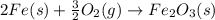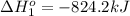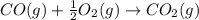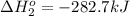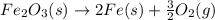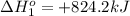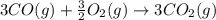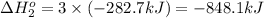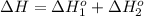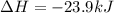Chemistry, 10.09.2019 02:30 lexibyrd120
Calculate δhrxn for the following reaction: fe₂o₃(s)+3co(g)→2fe(s)+3co₂(g) use the following reactions and given δh′s. 2fe(s)+3/2o₂(g)→fe₂o₃(s), δh = -824.2 kj co(g)+1/2o₂(g)→co₂(g), δh = -282.7 kj

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 23:00
What is the maximum amount of al2(so4)3 which could be formed from 15.84 g of al and 12.89 g of cuso4?
Answers: 2

Chemistry, 22.06.2019 04:00
Which of the following ocean acidification? are the most likely side effects of a ph less than 7.0 in the ocean b. more metal salts altering the ocean chemistry c. dissolution of the shells of marine organisms d. both a & b e. all of the above.
Answers: 3

Chemistry, 22.06.2019 11:00
3) in peaches, [oh]=3.16x10-11 m a) find [h+ ] b) what is the ph? c) is the solution acidic, basic, or neutral?
Answers: 1

Chemistry, 23.06.2019 04:20
Calculate the mass of 0.750 mol of the following substance. na3po4.
Answers: 1
You know the right answer?
Calculate δhrxn for the following reaction: fe₂o₃(s)+3co(g)→2fe(s)+3co₂(g) use the following reacti...
Questions

English, 29.11.2021 19:20




English, 29.11.2021 19:20


Mathematics, 29.11.2021 19:20

Mathematics, 29.11.2021 19:20







Chemistry, 29.11.2021 19:20

Computers and Technology, 29.11.2021 19:20


Mathematics, 29.11.2021 19:30

Mathematics, 29.11.2021 19:30