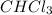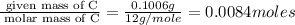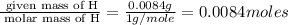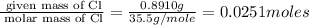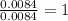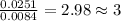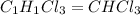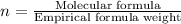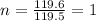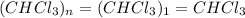Chemistry, 10.09.2019 01:10 gomezjuana123
Acompound was found to contain 10.06% carbon, 89.10% chlorine, and 0.84% hydrogen, by mass. if the molar mass of the compound was found to be 119.6 g/mol, its molecular formula will be

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:00
Fission of uranium-235 products energy and a. isotopes of smaller elements b. isotopes of larger elements c. lighter isotopes of uranium d. heavier isotopes of uranium
Answers: 3

Chemistry, 21.06.2019 22:00
During chemistry class, carl performed several lab tests on two white solids. the results of three tests are seen in the data table. based on this data, carl has concluded that substance b must have bonds.
Answers: 2

Chemistry, 21.06.2019 23:00
What is the molecular formula for a compound that is 46.16% carbon, 5.16% hydrogen, and 48.68% fluorine? the molar mass of the compound is 156.12 g/mol
Answers: 2

Chemistry, 21.06.2019 23:00
Plz choose one of the compounds from the table and explain how you know the numbers of atoms in your formula. is it possible for two different compounds to be made from the exact same two elements? why or why not? with a limited number of elements (less than 120 are known), does this mean we also have a small number of compounds or do we have a large number of compounds in this world?
Answers: 1
You know the right answer?
Acompound was found to contain 10.06% carbon, 89.10% chlorine, and 0.84% hydrogen, by mass. if the m...
Questions




Mathematics, 27.01.2021 14:00


Biology, 27.01.2021 14:00


Business, 27.01.2021 14:00

Mathematics, 27.01.2021 14:00



Mathematics, 27.01.2021 14:00

Business, 27.01.2021 14:00

Social Studies, 27.01.2021 14:00

History, 27.01.2021 14:00


English, 27.01.2021 14:00

SAT, 27.01.2021 14:00

English, 27.01.2021 14:00