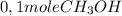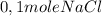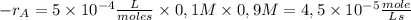Chemistry, 10.09.2019 00:20 disneyshree9427
The reaction described by the equation ch 3 cl + naoh → ch 3 oh + nacl follows the second-order rate law, rate = k [ ch 3 cl ] [ naoh ] . when this reaction is carried out with starting concentrations [ ch 3 cl ] = 0.2 m and [ naoh ] = 1.0 m , the measured rate is 1 × 10 − 4 mol l − 1 s − 1 . what is the rate after one-half of the ch 3 cl has been consumed? (caution: the initial concentrations of the starting materials are not identical in this experiment. hint: determine how much of the naoh has been consumed at this point and what its new concentration is, compared with its initial concentration.)

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 19:00
Dying the folding patterns of protein molecules can microbiologists better understand cellular processes as well as some diseases, such as alzheimer’s, that are caused by proteins that have misfolded. the folding of these complicated molecules can be simulated on computers, but it takes a lot of processor power and time for even expensive supercomputers to do this. a group of researchers at stanford university developed software that can be used to distribute the processing of data to anyone who is willing to donate time on their idle personal computers. as a result, the researchers have been able to achieve protein-folding simulations that are far better than those other computing methods have done. which statement best describes the work of these researchers? the work is not scientific because the data are not processed in one location. the work is not scientific because the simulations are not reproducible. the researchers applied creativity to solve a problem in running an experiment. the researchers used only well-established scientific techniques.
Answers: 3

Chemistry, 22.06.2019 13:50
What happens when an atom of sulfur combines with two atoms of chlorine to produce sci2? a. each chlorine atom shares a pair of electrons with the sulfur atom. b. an electron is transferred from each chlorine atom to the sulfur atom. c. an electron is transferred from the sulfur atom to each chlorine atom. d. each chlorine atom shares all its valence electrons with the sulfur atom.
Answers: 2

Chemistry, 22.06.2019 17:00
According to the kinetic-molecular theory, what happens to a liquid when it is transferred from one container to another? the volume and the shape stay the same. the volume increases to fill the new container, but the shape stays the same. the volume stays the same, but the shape changes to fit the new container. the volume and the shape change to fill the new container.
Answers: 2

Chemistry, 23.06.2019 00:40
To prevent the presence of air, noble gases are placed over highly reactive chemicals to act as inert "blanketing" gases. a chemical engineer places a mixture of noble gases consisting of 4.37 g of he, 13.36 g of ne, and 36.65 g of kr in a piston-cylinder assembly at stp. calculate the partial pressure in torr of kr.
Answers: 1
You know the right answer?
The reaction described by the equation ch 3 cl + naoh → ch 3 oh + nacl follows the second-order rate...
Questions

English, 05.03.2020 01:58

History, 05.03.2020 01:59






Mathematics, 05.03.2020 02:03




Spanish, 05.03.2020 02:03

Spanish, 05.03.2020 02:03




Computers and Technology, 05.03.2020 02:04



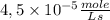
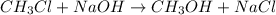
![-r_{A}=k \times [CH_{3}Cl] \times [NaOH]](/tpl/images/0226/2359/ac51f.png)
![1 \times 10^{-4}\frac{mole}{Ls}=k \times [0,2M] \times [1,0M] =5 \times 10^{-4}\frac{L}{mole s}](/tpl/images/0226/2359/a3f49.png)
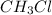 is consumed the mixture is composed by
is consumed the mixture is composed by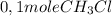 (half is consumed)
(half is consumed)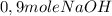 (by stoicheometry)
(by stoicheometry)