
Chemistry, 09.09.2019 22:30 sarahlearn3
Calculate the osmotic pressure (in torr) of 6.00 l of an aqueous 0.958 m solution at 30.°c, if the solute concerned is totally ionized into three ions (e. g., it could be na2so4 or mgcl2).

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 07:30
Free answer. the treaty of versailles ended world war i, but some of the terms of the treaty contributed to the beginning of world war ii. which was one of the terms of the treaty? the answer would be "germany was forces to pay reparations to the allied countries.". i hope this .
Answers: 1


Chemistry, 23.06.2019 00:00
This statement about matter and its behavior is best classified as a
Answers: 1

You know the right answer?
Calculate the osmotic pressure (in torr) of 6.00 l of an aqueous 0.958 m solution at 30.°c, if the s...
Questions









Mathematics, 25.07.2019 18:10


English, 25.07.2019 18:10

History, 25.07.2019 18:10

Mathematics, 25.07.2019 18:10




English, 25.07.2019 18:10



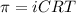
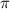 = osmotic pressure of the solution = ?
= osmotic pressure of the solution = ?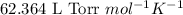
![30^oC=[30+273]K=303K](/tpl/images/0226/1460/fd4b3.png)



