
Chemistry, 09.09.2019 22:10 fiorentinologan4
The haber-bosch process is a very important industrial process. in the haber-bosch process, hydrogen gas reacts with nitrogen gas to produce ammonia according to the equation 3h2(g)+n2(g)→2nh3(g) the ammonia produced in the haber-bosch process has a wide range of uses, from fertilizer to pharmaceuticals. however, the production of ammonia is difficult, resulting in lower yields than those predicted from the chemical equation. 1.15 g h2 is allowed to react with 9.93 g n2, producing 1.12 g nh3. part a what is the theoretical yield in grams for this reaction under the given conditions? express your answer to three significant figures and include the appropriate units.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 08:00
An electron moved from shell n = 2 to shell n = 1. what most likely happened during the transition? a fraction of a photon was added. a photon of energy was absorbed. a fraction of a photon was removed. a photon of energy was released.
Answers: 1

Chemistry, 22.06.2019 16:30
4. a 20-kg child is tossed up into the air by her parent. the child is 2 meters off the ground traveling 5 m/s. circle one: ke / gpe / both show your work for finding the values of each type of energy the object has:
Answers: 1

Chemistry, 23.06.2019 02:00
What is the source of continuous heat and energy that we receive from the sun
Answers: 2

Chemistry, 23.06.2019 06:40
A250 g sample of water with an initial temperatureof 98.8 closes 6500 joules of heat. what is the finaltemperature of the water?
Answers: 1
You know the right answer?
The haber-bosch process is a very important industrial process. in the haber-bosch process, hydrogen...
Questions


Mathematics, 23.02.2022 14:00

Geography, 23.02.2022 14:00




SAT, 23.02.2022 14:00






Biology, 23.02.2022 14:00



English, 23.02.2022 14:00




Mathematics, 23.02.2022 14:00

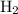 and
and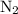 .
.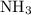 will be produced?
will be produced?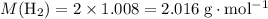 .
.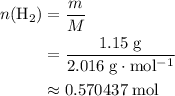 .
.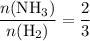 .
.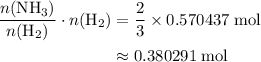 .
.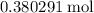 of
of 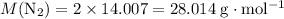 .
.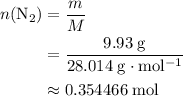 .
.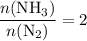 .
.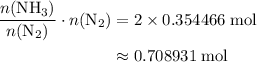 .
.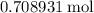 of
of 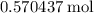 of
of 

