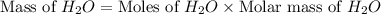Chemistry, 09.09.2019 19:30 Frenchfries13
Consider the following unbalanced chemical equation. c5h12(l) + o2(g) → co2(g) + h2o(l) if 21.9 grams of pentane (c5h12) are burned in excess oxygen, how many grams of h2o will be produced?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 06:30
Predict whether the changes in enthalpy, entropy, and free energy will be positive or negative for the boiling of water, and explain your predictions. how does temperature affect the spontaneity of this process?
Answers: 1

Chemistry, 22.06.2019 13:00
Using the thermodynamic information in the aleks data tab, calculate the standard reaction free energy of the following chemical reaction: →+p4o10s6h2ol4h3po4s round your answer to zero decimal places.
Answers: 3

Chemistry, 22.06.2019 17:30
The polymer used for the nonstick surface of cooking utensils is 24.0%c and 76%f by mass. what is the empirical formula of this polymer?
Answers: 2

Chemistry, 22.06.2019 18:10
The atom fluorine generally will become stable through the formation of an ionic chemical compound by accepting electron(s) from another atom. this process will fill its outer energy level of electrons.
Answers: 1
You know the right answer?
Consider the following unbalanced chemical equation. c5h12(l) + o2(g) → co2(g) + h2o(l) if 21.9 gram...
Questions


Mathematics, 18.03.2021 01:40



English, 18.03.2021 01:40






Mathematics, 18.03.2021 01:40

Mathematics, 18.03.2021 01:40



Mathematics, 18.03.2021 01:40





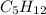 = 21.9 g
= 21.9 g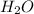 = 18 g/mole
= 18 g/mole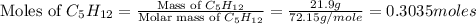
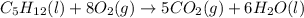
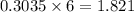 moles of
moles of