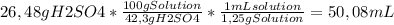Chemistry, 09.09.2019 19:10 runaway173
50 ml of a mixture consisting of 0.019 m cerium (iv) and 2.7 m h2so4, or sulfuric acid. begin by preparing 100 ml of 2.7 m h2so4 using the available solution of 42.3% w/w h2so4. this concentration unit, which may be less familiar to you, is a weight-to-weight percent (100.0 g of the solution contains 42.3 g of h2so4). the density of 42.3% w/w h2so4 is 1.25 g solution/ml solution. using a graduated cylinder, measure out the correct volume of 42.3% w/w h2so4 and slowly add it to a 100-ml volumetric flask that already contains approximately 25 ml of deionized water.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 14:00
Which of the following best defines homeostasis? forming identical cells breaking down glucose maintaining stable internal conditions increasing an organism's temperature
Answers: 3

Chemistry, 23.06.2019 12:00
Which element has the largest atomic radius? a. asb. nc. pd. sb
Answers: 2

Chemistry, 23.06.2019 16:20
What is the concentration, in grams per liter, of a solution prepared by dissolving 0.00040 mol hcl in 2.2 l h2o? assume that the volume of the solution does not change when the hcl is added.
Answers: 1

Chemistry, 23.06.2019 20:00
Assume you inhale in about 6 l of air per minute how much nitrogen do you breathe in one minute
Answers: 1
You know the right answer?
50 ml of a mixture consisting of 0.019 m cerium (iv) and 2.7 m h2so4, or sulfuric acid. begin by pre...
Questions



Health, 14.07.2019 03:00

History, 14.07.2019 03:00

Biology, 14.07.2019 03:00





Biology, 14.07.2019 03:00


Mathematics, 14.07.2019 03:00

Social Studies, 14.07.2019 03:00