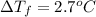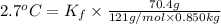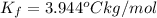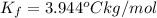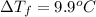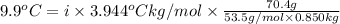Chemistry, 09.09.2019 18:30 chloejaylevesque
When 70.4 g of benzamide (c, h,no) are dissolved in 850. g of a certain mystery liquid x, the freezing point of the solution is 2.7 c lower than the freezing point of pure x. on the other hand, when 70.4 g of ammonium chloride (nh ci) are dissolved in the same mass of x, the freezing point of the solution is 9.9 °c lower than the freezing point of pure x. calculate the van't hoff factor for ammonium chloride in x. be sure your answer has a unit symbol, if necessary, and round your answer to 2 significant digits.

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 04:30
In which phase(s) do the molecules take the shape of the container?
Answers: 1

Chemistry, 22.06.2019 10:30
Earth's axis of rotation is tilted at an angle of 23.5 degrees. what is one change you would see on earth if its axis was not tilted?
Answers: 3

Chemistry, 22.06.2019 12:30
Sodium sulfate dissolves as follows: na2so4(s) → 2na+(aq) + so42- (aq). how many moles of na2so4 are required to make 1.0 l of solution in which the na concentration is 0.10 m?
Answers: 2
You know the right answer?
When 70.4 g of benzamide (c, h,no) are dissolved in 850. g of a certain mystery liquid x, the freezi...
Questions

History, 25.10.2019 01:43


Chemistry, 25.10.2019 01:43




English, 25.10.2019 01:43


Spanish, 25.10.2019 01:43

Chemistry, 25.10.2019 01:43

History, 25.10.2019 01:43



Social Studies, 25.10.2019 01:43

Social Studies, 25.10.2019 01:43


Mathematics, 25.10.2019 01:43



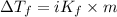
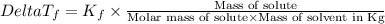 ...(1)
...(1)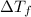 =Elevation in boiling point =
=Elevation in boiling point = 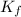 = Freezing point constant
= Freezing point constant