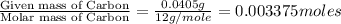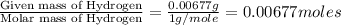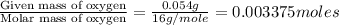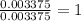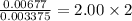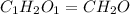Chemistry, 09.09.2019 16:10 batoolishak7475
A0.1014 g sample of a purified compound containing c, h, and, o was burned in a combustion apparatus and produced 0.1486 g co2 and 0.0609 g of h2o. what is the empirical formula of this compound?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 08:30
Which metal exist in liquid state and can be cut with knife ?
Answers: 2

Chemistry, 22.06.2019 12:10
Achemistry student needs to standardize a fresh solution of sodium hydroxide. he carefully weighs out of oxalic acid , a diprotic acid that can be purchased inexpensively in high purity, and dissolves it in of distilled water. the student then titrates the oxalic acid solution with his sodium hydroxide solution. when the titration reaches the equivalence point, the student finds he has used of sodium hydroxide solution.calculate the molarity of the student's sodium hydroxide solution. be sure your answer has the correct number of significant digits.
Answers: 1

Chemistry, 22.06.2019 15:00
‘which reaction would most likely require the use of an inert electrode?
Answers: 1

Chemistry, 22.06.2019 16:00
What rule is used to determine how many covalent bonds an element can form? a. the number of covalent bonds is equal to six c the number of covalent bonds is equal to five minus the group number plus the group number b. the number of covalent bonds is equal to eight d. none of the above minus the group number select the best answer from the choices provided
Answers: 2
You know the right answer?
A0.1014 g sample of a purified compound containing c, h, and, o was burned in a combustion apparatus...
Questions


English, 10.01.2022 14:40

Mathematics, 10.01.2022 14:40

Mathematics, 10.01.2022 14:40

Physics, 10.01.2022 14:40


Mathematics, 10.01.2022 14:40

Mathematics, 10.01.2022 14:40

Mathematics, 10.01.2022 14:50






History, 10.01.2022 14:50

Mathematics, 10.01.2022 14:50

Mathematics, 10.01.2022 14:50



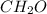
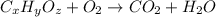
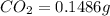
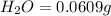
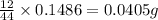 of carbon will be contained.
of carbon will be contained.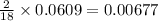 of hydrogen will be contained.
of hydrogen will be contained.