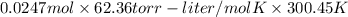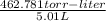Chemistry, 07.09.2019 05:30 dogsarecute278
The vapor pressure of a volatile liquid can be determined by slowly bubbling a known volume of gas through it at a known temperature and pressure. in an experiment, 5.01 l of n2 gas is passed through 7.9286 g of liquid benzene, c6h6, at 27.3 ∘c and atmospheric pressure. the liquid remaining after the experiment weighs 5.9987 g . assuming that the gas becomes saturated with benzene vapor and that the total gas volume and temperature remain constant, what is the vapor pressure of the benzene in torr?

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 04:00
What layer of the atmosphere is directly above the troposphere?
Answers: 1

Chemistry, 22.06.2019 04:00
Asample of aluminum foil contains 8.60 × 1023 atoms. what is the mass of the foil?
Answers: 1

Chemistry, 22.06.2019 07:10
An experimental procedure requires a 10 ml of acid to be dissolved
Answers: 2
You know the right answer?
The vapor pressure of a volatile liquid can be determined by slowly bubbling a known volume of gas t...
Questions


World Languages, 12.11.2020 19:10

Social Studies, 12.11.2020 19:10

Mathematics, 12.11.2020 19:10



English, 12.11.2020 19:10

Mathematics, 12.11.2020 19:10


Mathematics, 12.11.2020 19:10

Mathematics, 12.11.2020 19:10



History, 12.11.2020 19:10

Computers and Technology, 12.11.2020 19:10

Mathematics, 12.11.2020 19:10

Social Studies, 12.11.2020 19:10



Mathematics, 12.11.2020 19:10

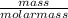
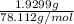
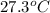 = 27.3 + 273.15 = 300.45 K
= 27.3 + 273.15 = 300.45 K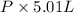 =
=