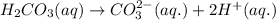Chemistry, 07.09.2019 04:30 alliemeade1
Which of the following are conjugate acid/base pairs? select all that apply.
hcooh and hcoo-
hno3and no3-
h2so4 and so42-
nacl and naoh
h2co3 and co32-

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Which type of bond is present in hydrogen sulfide (h2s)? the table of electronegativities is given. a. hydrogen b. ionic c. nonpolar covalent d. polar covalent
Answers: 1

Chemistry, 22.06.2019 03:30
If a solution is considered basic, then a) the hydroxide ion and hydronium ion concentrations are equal. b) the hydroxide ion concentration is less than the hydronium ion concentration. c) the hydronium ion concentration is greater than the hydroxide ion concentration. d) the hydroxide ion concentration is greater than the hydronium ion concentration.
Answers: 1

Chemistry, 22.06.2019 04:00
4. absorption has the highest risk of overdose due to increased potency. a. rectal b. oral c. transdermal d. intranasal
Answers: 2

Chemistry, 22.06.2019 18:30
Which of the following words describe the reality that the universe looks the same from various perspective
Answers: 3
You know the right answer?
Which of the following are conjugate acid/base pairs? select all that apply.
hcooh and hcoo-<...
hcooh and hcoo-<...
Questions

English, 30.09.2019 22:30

Biology, 30.09.2019 22:30

Physics, 30.09.2019 22:30

Advanced Placement (AP), 30.09.2019 22:30

History, 30.09.2019 22:30

Biology, 30.09.2019 22:30

History, 30.09.2019 22:30

Mathematics, 30.09.2019 22:30

Arts, 30.09.2019 22:30

English, 30.09.2019 22:30

Mathematics, 30.09.2019 22:30

English, 30.09.2019 22:30


Computers and Technology, 30.09.2019 22:30

Mathematics, 30.09.2019 22:30


History, 30.09.2019 22:30



Biology, 30.09.2019 22:30

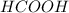 and
and 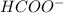
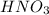 and
and 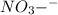
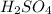 and
and 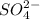
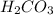 and
and 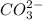
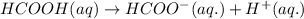
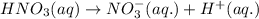
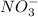 which is a conjugate base.
which is a conjugate base.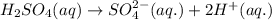
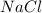 and
and 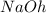 are not conjugate acid/ base pairs.
are not conjugate acid/ base pairs.