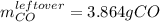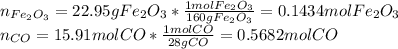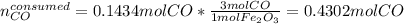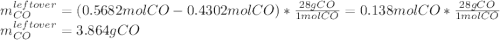Chemistry, 07.09.2019 04:30 TightKnowsDaWhey
Iron(iii) oxide reacts with carbon monoxide according to the equation: fe2o3(s) + 3 co(g) → 2 fe(s) + 3 co2(g) a reaction mixture initially contains 22.95 g fe2o3 and 15.91 g co. assume that the reaction will progress to 100% completion. what mass (in g) of the excess reactant is leftover?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 10:00
Suppose the universe were completely empty except for one object-a solid sphere moving through space of 100 km/s. what sort of path would the object be moving in? explain your answer
Answers: 1

Chemistry, 22.06.2019 19:00
Sum of brother and sisters age is 26. four times the brothers age is subtracted from three times the sisters age, the difference is 8. what are the ages of the brother and sister?
Answers: 1

Chemistry, 22.06.2019 22:30
Which of the following is not an assumption that scientists must make about the natural world? a. regularity b. causality c. predictability d. plausibility
Answers: 1

Chemistry, 23.06.2019 01:20
How can parts of a solution be separated by chromatography?
Answers: 1
You know the right answer?
Iron(iii) oxide reacts with carbon monoxide according to the equation: fe2o3(s) + 3 co(g) → 2 fe(s)...
Questions

Physics, 04.11.2019 17:31

Mathematics, 04.11.2019 17:31




Physics, 04.11.2019 17:31

Chemistry, 04.11.2019 17:31


Computers and Technology, 04.11.2019 17:31


Mathematics, 04.11.2019 17:31


Health, 04.11.2019 17:31


English, 04.11.2019 17:31

Biology, 04.11.2019 17:31