
Chemistry, 07.09.2019 03:30 grenades5027
Calculate the molar solubility of pbbr2 (ksp = 4.67x10-6) in 0.10m nabr solution.

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 13:00
What happens to the average kinetic energy of a gas when the particles of the gas collide against each other at a constant temperature and volume? explain your answer.
Answers: 3

Chemistry, 22.06.2019 22:30
Which process describes vaporization that takes place below the surface of a liquid? condensation melting boiling evaporation
Answers: 1

Chemistry, 22.06.2019 23:00
What is the solubility-product constant of barium sulfate, baso4, if a saturated solution is 1.03 ´ 10-5 m?
Answers: 3
You know the right answer?
Calculate the molar solubility of pbbr2 (ksp = 4.67x10-6) in 0.10m nabr solution....
Questions



Spanish, 19.12.2020 08:30



Mathematics, 19.12.2020 08:30

History, 19.12.2020 08:30

Biology, 19.12.2020 08:30

Mathematics, 19.12.2020 08:30


Spanish, 19.12.2020 08:30



Social Studies, 19.12.2020 08:30

World Languages, 19.12.2020 08:30

History, 19.12.2020 08:30


Biology, 19.12.2020 08:30


History, 19.12.2020 08:30

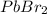 will dissociate into ions as follows.
will dissociate into ions as follows.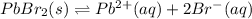
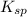 for this reaction will be as follows.
for this reaction will be as follows.![K_{sp} = [Pb^{2+}][Br^{-}]^{2}](/tpl/images/0225/0552/a1fd6.png)
![[Pb^{2+}]](/tpl/images/0225/0552/0acfd.png) =
= ![[Pb^{2+}]_{o}](/tpl/images/0225/0552/46ef8.png) + x
+ x![[Br^{-}]^{2}](/tpl/images/0225/0552/1eb91.png) =
= ![[Br^{-}]_{o}](/tpl/images/0225/0552/89ded.png) + 2x
+ 2x![([Pb^{2+}]_{o} + x)([Br^{-}]_{o} + 2x)^{2}](/tpl/images/0225/0552/3053a.png)
![[Br^{-}]_{o} + 2x](/tpl/images/0225/0552/19f1d.png) will approximately equals to
will approximately equals to ![K_{sp} = x[Br^{-}]^{2}_{o}](/tpl/images/0225/0552/ae97c.png)
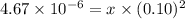
 M
M

