

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 13:00
If two objects at different te,peraure are in contact with each other what happens to their temperature
Answers: 1

Chemistry, 23.06.2019 06:30
Acompound has the molecular formula c3h8. which class of organic compounds does it belong to?
Answers: 2

Chemistry, 23.06.2019 07:30
Type the letter that represents the correct location for each particle type below. the neutron is found at __ the electron is found at __ the proton is found at __
Answers: 2
You know the right answer?
Ahf [no2] = +33.2 kj mol. use the values below to calculate the standard molar enthalpy change for t...
Questions

Mathematics, 25.05.2021 19:00

Chemistry, 25.05.2021 19:00

Computers and Technology, 25.05.2021 19:00



World Languages, 25.05.2021 19:00


Biology, 25.05.2021 19:00

French, 25.05.2021 19:00

Business, 25.05.2021 19:00


Mathematics, 25.05.2021 19:00

Mathematics, 25.05.2021 19:00


Mathematics, 25.05.2021 19:00



Mathematics, 25.05.2021 19:00


Mathematics, 25.05.2021 19:00

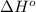
![\Delta H^o_{rxn}=\sum [n\times \Delta H^o_f(product)]-\sum [n\times \Delta H^o_f(reactant)]](/tpl/images/0224/9686/45485.png)
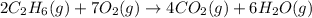
![\Delta H^o_{rxn}=[(4\times \Delta H^o_f_{(CO_2)})+(6\times \Delta H^o_f_{(H_2O)})]-[(2\times \Delta H^o_f_{(C_2H_6)})+(7\times \Delta H^o_f_{(O_2)})]](/tpl/images/0224/9686/92f41.png)
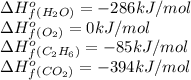
![\Delta H^o_{rxn}=[(4\times (-394))+(6\times (-286))]-[(2\times (-85))+(7\times 0)]\\\\\Delta H^o_{rxn}=-3122kJ](/tpl/images/0224/9686/cf6bf.png)


