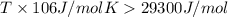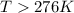Chemistry, 06.09.2019 22:30 muanghoih14
For a reaction for which ah = +29.3 kj/mol and as = +106 j/mol•k, which of the following statements is true? a) the reaction is spontaneous above 276 k. b) the reaction is spontaneous below 276 k. c) the reaction will never reach equilibrium. d) the reaction will never be spontaneous.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 19:20
Anyone who's in connections academy chemistry b have the factors that affect the rate of a reaction portfolio already done?
Answers: 3

Chemistry, 22.06.2019 21:30
Electromagnets coils of wire paper clips picked up 10 3 15 6 20 9 25 12 ms. owens' class was studying magnets. ms. owens showed her students how to make an electromagnet using a nail, a d-cell battery, and plastic coated wire. the students wrapped the wire around the nail and then attached the ends to the battery. when they were finished, they tested their magnets by investigating how many paperclips their magnets could pick up. they also tested whether they could increase the strength of their electromagnets by using more coils of wire. they recorded the class average of their results in the data table seen here. ms. owens asked her students to graph their data in a line graph. how should the students label the x-axis on their line graph? a) size of battery b) number of paper clips c) number of coils of wire d) strength of electromagnet
Answers: 2

Chemistry, 23.06.2019 00:30
•hydration •dissociation •dissolving which one goes to which
Answers: 1

You know the right answer?
For a reaction for which ah = +29.3 kj/mol and as = +106 j/mol•k, which of the following statements...
Questions


Biology, 20.11.2020 18:40





Mathematics, 20.11.2020 18:40

Social Studies, 20.11.2020 18:40

Mathematics, 20.11.2020 18:40

Mathematics, 20.11.2020 18:40


Biology, 20.11.2020 18:40




Mathematics, 20.11.2020 18:40

Mathematics, 20.11.2020 18:40

History, 20.11.2020 18:40

Mathematics, 20.11.2020 18:40

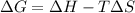
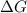 = Gibbs free energy
= Gibbs free energy 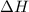 = enthalpy change = +29.3 kJ/mol =29300 J/mol
= enthalpy change = +29.3 kJ/mol =29300 J/mol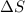 = entropy change = +106 J/molK
= entropy change = +106 J/molK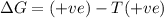
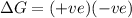
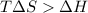 for reaction to be spontaneous
for reaction to be spontaneous