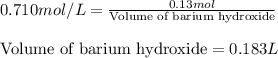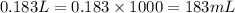Chemistry, 06.09.2019 22:10 zmoore8015
Calculate the number of milliliters of 0.710 m ba(oh)2 required to precipitate all of the mn2+ ions in 161 ml of 0.796 m kmno4 solution as mn(oh)2. the equation for the reaction is: mnso4(aq) + ba(oh)2(aq) mn(oh)2(s) + baso4(aq)

Answers: 3


Another question on Chemistry




Chemistry, 22.06.2019 12:40
In the following table, all the columns for the element calcium are filled out correctly. element electron structure of atom electron structure of ion net ionic charge calcium 1s22s22p63s23p64s2 1s32s22p63s23p64s1 +1 true false
Answers: 2
You know the right answer?
Calculate the number of milliliters of 0.710 m ba(oh)2 required to precipitate all of the mn2+ ions...
Questions


Social Studies, 11.07.2019 04:30

History, 11.07.2019 04:30


Spanish, 11.07.2019 04:30



History, 11.07.2019 04:30









Social Studies, 11.07.2019 04:30



History, 11.07.2019 04:30

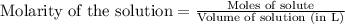 .....(1)
.....(1)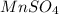 = 0.796 M
= 0.796 M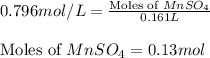
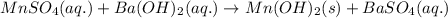
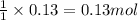 of barium hydroxide
of barium hydroxide