
Chemistry, 06.09.2019 20:30 silvajimena74
Enter your answer in the provided box. the atomic masses of 63cu (69.17 percent) and 65cu (30.83 percent) are 62.94 and 64.93 amu, respectively. calculate the average atomic mass of copper. the percentages in parentheses denote the relative abundances.

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 10:00
The tendency of water molecules to stick together is referred to as a) adhesion b) polarity c) cohesion d) transpiration e) evaporation
Answers: 1

Chemistry, 22.06.2019 11:50
Calculate the molarity of each of the following solutions. part a) 0.12 mol of lino3 in 5.5 l of solution part b) 60.7 g c2h6o in 2.48 l of solution part c) 14.2 mg ki in 100 ml of solution
Answers: 2

Chemistry, 22.06.2019 14:00
Displacement is the slope of a velocity vs. time graph a. true b. false
Answers: 1
You know the right answer?
Enter your answer in the provided box. the atomic masses of 63cu (69.17 percent) and 65cu (30.83 per...
Questions


English, 25.11.2020 01:00


Mathematics, 25.11.2020 01:00




Mathematics, 25.11.2020 01:00



History, 25.11.2020 01:00

Biology, 25.11.2020 01:00


English, 25.11.2020 01:00

English, 25.11.2020 01:00


Mathematics, 25.11.2020 01:00

Mathematics, 25.11.2020 01:00

Mathematics, 25.11.2020 01:00

Mathematics, 25.11.2020 01:00

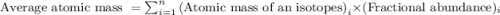 .....(1)
.....(1)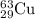 isotope:
isotope: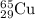 isotope:
isotope:![\text{Average atomic mass of Copper}=[(62.94\times 0.6917)+(64.93\times 0.3083)]\\\\\text{Average atomic mass of copper}=63.55amu](/tpl/images/0224/6800/fff18.png)


