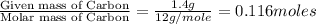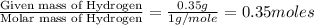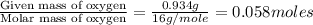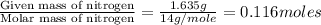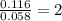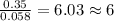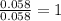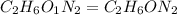Dimethyl nitrosamine is a known carcinogen. it can be formed in the intestinal tract when digestive juices react with the nitrite ion is preserved and smoked meats. it is made up of carbon, hydrogen, nitrogen, and oxygen atoms. a 4.319 g sample of dimethyl nitrosamine burned in oxygen yields 5.134 g of co2 and 3.173 g of h2 o. the compound contains 37,82% by mass of nitrogen. what is the empirical formula of dimethyl nitrosamine?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:30
The first element on the periodic table of elements is carbon. a. true b. false
Answers: 2

Chemistry, 21.06.2019 23:50
2points what is the job of a scientist? a. to answer ethical questions. b. to write laws based on his or her knowledge. c. to ask and answer scientific questions. d. to ignore facts that do not support his or her theory.
Answers: 1

Chemistry, 22.06.2019 01:30
An empty fuel tank can still contain and therefore can be even more dangerous than one full of liquid fuel.
Answers: 1

Chemistry, 22.06.2019 23:00
How does the value of the equilibrium constant show that a reaction reaches equilibrium very quickly? (a) the equilibrium constant is large. (b) the equilibrium constant is small. (c) the equilibrium constant is zero. (d) the value of the equilibrium constant does not show how quickly a reaction comes to equilibrium.
Answers: 1
You know the right answer?
Dimethyl nitrosamine is a known carcinogen. it can be formed in the intestinal tract when digestive...
Questions


Mathematics, 22.06.2019 10:00



Mathematics, 22.06.2019 10:00



Mathematics, 22.06.2019 10:00

History, 22.06.2019 10:00

Mathematics, 22.06.2019 10:00


Mathematics, 22.06.2019 10:00






History, 22.06.2019 10:00


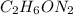
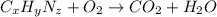
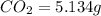
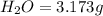
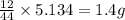 of carbon will be contained.
of carbon will be contained.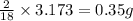 of hydrogen will be contained.
of hydrogen will be contained.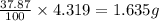 of nitrogen
of nitrogen