
Chemistry, 06.09.2019 18:10 itsyagirl11076
For the overall reaction below, which of the following is the correctly written rate law? overall reaction: o3(g)+2no2(g)→n2o5(g)+o2(g) step 1: o3(g)+no2(g)→no3(g)+o2(g) slow step 2: no3(g)+no2(g)→n2o5(g) fast view available hint(s) for the overall reaction below, which of the following is the correctly written rate law? overall reaction: step 1: slow step 2: fast rate=k[o3][no2]2 rate=k[no3][no2] rate=k[o3][no2] rate=k[o3][no2]2[n2o5][o2]

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:30
In order to calculate the amount of heat transferred you must know the __ and specific heat of the material, as well as the change in temperature. a. volume b. density c. mass d. enthalpy
Answers: 1

Chemistry, 22.06.2019 11:00
When hydrochloric acid reacts with potassium hydroxide solution, the following reaction occurs. hcl (aq) + koh (aq) h2o (l) + kcl (aq) the reaction gives off heat energy, so it is an reaction.
Answers: 1


Chemistry, 23.06.2019 01:00
Substance 33°f 100°f peanut oil solid liquid margarine solid liquid chocolate chips solid liquid which conclusion fits the data in the table? a. heat chemically changes chocolate and margarine. b. all solids become liquid at 100°f. c. removing heat from a substance it to melt. d. matter may change shape when it is heated.
Answers: 1
You know the right answer?
For the overall reaction below, which of the following is the correctly written rate law? overall r...
Questions

History, 12.01.2020 03:31

Mathematics, 12.01.2020 03:31


Mathematics, 12.01.2020 03:31




Biology, 12.01.2020 03:31

Mathematics, 12.01.2020 03:31


Mathematics, 12.01.2020 03:31


Mathematics, 12.01.2020 03:31

English, 12.01.2020 03:31

Mathematics, 12.01.2020 03:31

History, 12.01.2020 03:31



Mathematics, 12.01.2020 03:31

![Rate=k[O_3][NO_2]^2](/tpl/images/0224/5146/9d0a3.png)
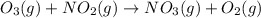 slow
slow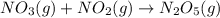 fast
fast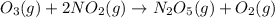
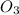 is 1 and Order with respect to
is 1 and Order with respect to 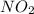 is 2.
is 2.

