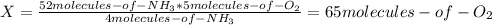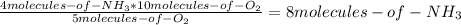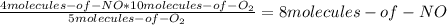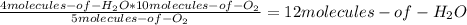Consider the following reaction: 4nh3(g) + 5o2(g) → 4no(g) + 6h2o(g) if a container were to have 10 molecules of o2 and 52 molecules of nh3 initially, how many total molecules (reactants plus products) would be present in the container after this reaction goes to completion?

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 15:30
The reactions of photosynthesis occur in the of plant cell? a.mitochondria. b. lysosomes. c. chloroplasts. d. chlorophyll
Answers: 1

Chemistry, 22.06.2019 21:30
Isopropyl alcohol, (ch3)2choh, is a common solvent. determine the percent by mass of hydrogen in isopropyl alcohol. a) 6.71% h b) 13.4% h c) 25.0% h d) 53.3% h
Answers: 1

Chemistry, 22.06.2019 23:30
With the largest atoms and the smallest number of valence electrons and with the smallest atoms and the greatest number of valence electrons are the most reactive. a. nonmetals; metals b. nonmetals; transition elements c. transition elements; metals d. metals; nonmetals
Answers: 3
You know the right answer?
Consider the following reaction: 4nh3(g) + 5o2(g) → 4no(g) + 6h2o(g) if a container were to have 10...
Questions

Health, 26.06.2019 07:00

Mathematics, 26.06.2019 07:00

Spanish, 26.06.2019 07:00

Mathematics, 26.06.2019 07:00



Mathematics, 26.06.2019 07:00




Mathematics, 26.06.2019 07:00

Social Studies, 26.06.2019 07:00


Mathematics, 26.06.2019 07:00

World Languages, 26.06.2019 07:00

Social Studies, 26.06.2019 07:00


Physics, 26.06.2019 07:00

Mathematics, 26.06.2019 07:00