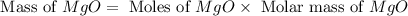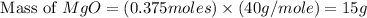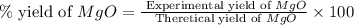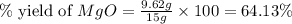Magnesium oxide can be made by heating magnesium metal in the presence of the oxygen. the balanced equation for the reaction is 2 mg(s) + o2(g) → 2 mgo(s) now consider that you react 10.0 g mg with 6.00 g o2 gas. if you were able to collect 9.62 g of mgo, what would be your percent yield for the reaction?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:00
Your friend offers to show you an intrusive igneous rock. which of the following would you expect to see?
Answers: 1

Chemistry, 22.06.2019 21:30
In science class richard learns that a substance has a boiling point of 230 fahrenheit his teacher ask him to convert this temperature to degrees celsius what is the boiling point of his substance in degrees celsius
Answers: 3

Chemistry, 23.06.2019 01:00
Reactions in cells take place at about a. 40°c b. 0° c. 100°c d. 60°c
Answers: 1

Chemistry, 23.06.2019 04:31
2ki + pb(no3)2 → 2kno3 + pbi2 determine how many moles of kno3 are created if 0.03 moles of ki are completely consumed.
Answers: 1
You know the right answer?
Magnesium oxide can be made by heating magnesium metal in the presence of the oxygen. the balanced e...
Questions

Mathematics, 09.11.2019 08:31

English, 09.11.2019 08:31




Chemistry, 09.11.2019 08:31



Mathematics, 09.11.2019 08:31


World Languages, 09.11.2019 08:31


Mathematics, 09.11.2019 08:31


Social Studies, 09.11.2019 08:31

Mathematics, 09.11.2019 08:31

History, 09.11.2019 08:31


Mathematics, 09.11.2019 08:31

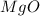 is, 64.13 %
is, 64.13 %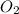 = 6 g
= 6 g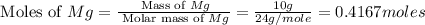
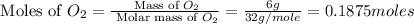
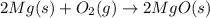
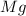
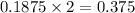 moles of
moles of