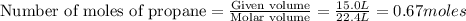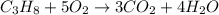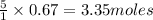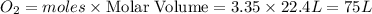Saved propane burns in air according to the equation c3ha(g 502lg)3co2) + 4h20(g) what volume of o2 in liters would be required if 15.0 l of propane burns, assuming that all of the gases are under the same conditions? short answer toolbar navigation e i e b ius ea this question will be sent to your instructor for grading. 20 of 25 l next > prev nere to search

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 11:30
What is the main reason why some developing countries fear the increase the free trade policies around the world?
Answers: 2

Chemistry, 22.06.2019 23:20
In medium-sized stars such as the sun, nuclear fusion almost always means the fusing of nuclei to form , but larger stars can produce elements as heavy as
Answers: 2

Chemistry, 23.06.2019 03:30
Astudent uses universal ph paper to find the ph of three solutions . solution a has a ph of 5 solution b has a ph of 11 and solution c has a ph of 7 identify which solution is acidic which solution is neutral and which solution is basic
Answers: 1

Chemistry, 23.06.2019 07:00
How does science use models to gain a better understanding of concepts?
Answers: 1
You know the right answer?
Saved propane burns in air according to the equation c3ha(g 502lg)3co2) + 4h20(g) what volume of o2...
Questions




History, 16.11.2020 16:50


Mathematics, 16.11.2020 16:50

English, 16.11.2020 16:50

Mathematics, 16.11.2020 16:50

Mathematics, 16.11.2020 16:50

Mathematics, 16.11.2020 16:50

Biology, 16.11.2020 16:50

Arts, 16.11.2020 16:50






Mathematics, 16.11.2020 16:50


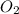 in liters would be required if 15.0 L of propane burns, assuming that all of the gases are under the same conditions.
in liters would be required if 15.0 L of propane burns, assuming that all of the gases are under the same conditions.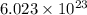 of particles.
of particles.