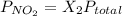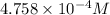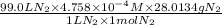Chemistry, 06.09.2019 16:20 cindyc1103
Calculate the mass of nitrogen dissolved at room temperature in an 99.0 l home aquarium. assume a total pressure of 1.0 atm and a mole fraction for nitrogen of 0.78. express your answer using two significant figures.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 03:40
Kc = 0.040 for the system below at 450oc. if a reaction is initiated with 0.40 mole of cl2 and 0.40 mole of pcl3 in a 2.0 liter container, what is the equilibrium concentration of cl2 in the same system? pcl5(g) ⇄ pcl3(g) + cl2(g)
Answers: 3

Chemistry, 22.06.2019 07:20
Why does his teacher ask him to balance the equation by including the correct coefficient
Answers: 1

Chemistry, 22.06.2019 08:30
If i initially have a gas at a pressure of 12 atm, a volume of 23 liters, and a temperature of 200 k, and then i raise the pressure to 14 atm and increase the temperature to 300 k, what is the new volume of the gas?
Answers: 1

You know the right answer?
Calculate the mass of nitrogen dissolved at room temperature in an 99.0 l home aquarium. assume a to...
Questions







Mathematics, 27.07.2020 02:01







Biology, 27.07.2020 02:01





Physics, 27.07.2020 02:01

 = 1 atm,
= 1 atm,  = 0.78
= 0.78