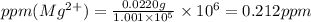Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 21:30
In one or two grammatically correct sentences, write a definition for the term molecule geometry
Answers: 3

Chemistry, 22.06.2019 23:30
The density of the solid phase of a substance is 0.90 g/cm3 and the density of the liquid phase is 1.0 g/cm3. a large increase in pressure will a. lower the freezing point b. raise the freezing point c. lower the boiling point d. raise the triple point e. lower the triple point
Answers: 1

Chemistry, 22.06.2019 23:30
If it is an isoelectronic series select true, if not select false. o2-, s2-, se2-, te2- na+, k+, rb+, cs+ n3-, p3-, as3-, sb3- ag, cd+, sn3+, sb4+ f-, cl-, br-, i- f-, ne, na+, mg2+ s2-, s, s6+
Answers: 1
You know the right answer?
Wastewater from a cement factory contains 0.280 g of ca2+ ion and 0.0220 g of mg2+ ion per 100.0 l o...
Questions


Biology, 24.03.2021 16:40

Mathematics, 24.03.2021 16:40

Mathematics, 24.03.2021 16:40




Mathematics, 24.03.2021 16:40



Mathematics, 24.03.2021 16:40

Mathematics, 24.03.2021 16:40

SAT, 24.03.2021 16:40

Mathematics, 24.03.2021 16:40






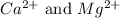 ions are 2.797 ppm and 0.212 ppm respectively.
ions are 2.797 ppm and 0.212 ppm respectively.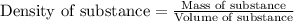
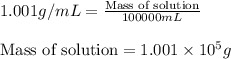
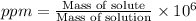
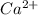 ions = 0.280 g
ions = 0.280 g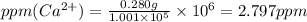
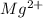 ions = 0.0220 g
ions = 0.0220 g