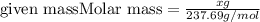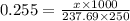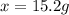Chemistry, 06.09.2019 04:10 valeriekbueno
How many grams of nickel (ii) chloride hexahydrate are required to prepare 250. ml of aqueous solution whose concentration is 0.255 m? the molar mass of nickel (ii) chloride hexahydrate is 237.69 g mol−1.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 18:50
Problem page gaseous ethane reacts with gaseous oxygen gas to produce gaseous carbon dioxide and gaseous water . if of water is produced from the reaction of of ethane and of oxygen gas, calculate the percent yield of water. be sure your answer has the correct number of significant digits in it.
Answers: 2

Chemistry, 22.06.2019 09:30
Which formula can be used to calculate the molar mass of hydrogen peroxide
Answers: 1

Chemistry, 22.06.2019 12:00
Consider the following reaction at equilibrium. 2co2 (g) 2co (g) + o2 (g) h° = -514 kj le châtelier's principle predicts that the equilibrium partial pressure of co (g) can be maximized by carrying out the reaction a. at high temperature and high pressure b. at high temperature and low pressure c. at low temperature and low pressure d. at low temperature and high pressure e. in the presence of solid carbon
Answers: 2

Chemistry, 22.06.2019 15:00
Which of the following is the correct formula for copper (i) sulfate trihydrate? cuso4 · 3h2o cuso4(h2o)3 cu2so4(h2o)3 cu2so4 · 3h2o
Answers: 1
You know the right answer?
How many grams of nickel (ii) chloride hexahydrate are required to prepare 250. ml of aqueous soluti...
Questions

Social Studies, 10.12.2019 04:31

Mathematics, 10.12.2019 04:31


Mathematics, 10.12.2019 04:31

Biology, 10.12.2019 04:31

History, 10.12.2019 04:31





History, 10.12.2019 04:31


Chemistry, 10.12.2019 04:31


Chemistry, 10.12.2019 04:31


Biology, 10.12.2019 04:31



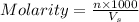
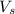 = volume of solution in ml
= volume of solution in ml