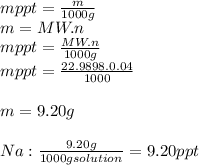
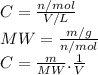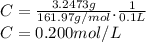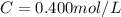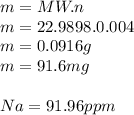3.2473 g of na2cro4 (mw = 161.97 g/mol) is dissolved in 100.0 ml of water. assuming the solution has a density of 1.00 g/ml, what is the concentration of na (mw = 22.9898 g/mol) in the solution in units of (a) molarity (m)? (b) parts per thousand (ppt)? (c) 10.0 ml of the solution is then diluted to a final volume of 1000.0 ml. what is the concentration of na in the diluted solution in units of parts per million (ppm)?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:30
Compare and contrast physical changes with chemical changes.
Answers: 1

Chemistry, 22.06.2019 09:00
Which two scientific disciplines are bridged by pharmaceutical drugs? chemistry and forensics chemistry and medicine biology and forensics biology and criminology
Answers: 3

Chemistry, 22.06.2019 21:00
Which of these is an example of pseudoscience? a) predicting the time of sunrise based on data on position of earth b) predicting the date of the moon phases based on data on position of earth c) predicting eclipses based on the position of the sun and the moon d) predicting future events in a person's life based on the position of the moon
Answers: 1

Chemistry, 22.06.2019 23:30
If maltose undergoes hydrolysis what subunits does it results to?
Answers: 2
You know the right answer?
3.2473 g of na2cro4 (mw = 161.97 g/mol) is dissolved in 100.0 ml of water. assuming the solution has...
Questions

English, 31.03.2020 21:58


Mathematics, 31.03.2020 21:58

Biology, 31.03.2020 21:58

Mathematics, 31.03.2020 21:58


English, 31.03.2020 21:58




Mathematics, 31.03.2020 21:59

Social Studies, 31.03.2020 21:59


Mathematics, 31.03.2020 21:59


Mathematics, 31.03.2020 21:59

Mathematics, 31.03.2020 21:59



Social Studies, 31.03.2020 21:59