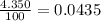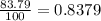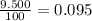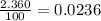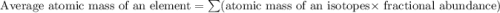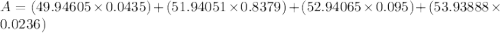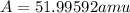Chemistry, 06.09.2019 02:10 josebienka
Acertain element x has four isotopes. 4.350% of x has a mass of 49.94605 amu. 83.79% of x has a mass of 51.94051 amu. 9.500% of x has a mass of 52.94065 amu. 2.360% of x has a mass of 53.93888 amu. what is the average atomic mass of element x?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 14:00
Which of the following statements is true? question 4 options: nuclear decay rates vary with the conditions of the reaction, but chemical reaction rates do not. chemical reaction rates vary with the conditions of the reaction, but nuclear decay rates do not. neither chemical reaction rates nor nuclear decay rates vary with the conditions of the reaction. both chemical reaction rates and nuclear decay rates vary with the conditions of the reaction.
Answers: 1

Chemistry, 21.06.2019 15:30
The isotonic saline solution described in part a is connected to an unknown solution via a semipermeable membrane, the unknown solution level drops. based on this information, what can be said about these two solutions?
Answers: 1

Chemistry, 22.06.2019 06:00
Calculate - analysis of compound composed of iron and oxygen yields 174.86 of fe and 75.14g of o. what is the empirical formula for this compound?
Answers: 3

You know the right answer?
Acertain element x has four isotopes. 4.350% of x has a mass of 49.94605 amu. 83.79% of x has a mass...
Questions


English, 04.12.2019 22:31



English, 04.12.2019 22:31

Chemistry, 04.12.2019 22:31







Biology, 04.12.2019 22:31

English, 04.12.2019 22:31




English, 04.12.2019 22:31


Mathematics, 04.12.2019 22:31