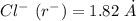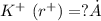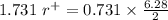Chemistry, 05.09.2019 23:30 Cartucho1978
The edge length of the unit cell of kcl (nacl-like structure, fcc) is 6.28 å. assuming anion-cation contact along the cell edge, calculate the radius of the potassium ion. the radius of the chloride ion is 1.82 å.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:10
The peak wavelength for the blackbody curve of a star is in the uv range. assuming the radiation from this star can reach earth, would you be able to see it?
Answers: 2

Chemistry, 22.06.2019 04:00
How do scientists think that gravity affected the formation of our solar system?
Answers: 1

Chemistry, 22.06.2019 11:50
Acompound has a molecular weight of 12.124 atomic mass units and the empirical formula c3h40. what is the molecular formula of the compound?
Answers: 3

Chemistry, 22.06.2019 12:30
What metric units would you use to measure the thickness of a key
Answers: 3
You know the right answer?
The edge length of the unit cell of kcl (nacl-like structure, fcc) is 6.28 å. assuming anion-cation...
Questions


English, 20.07.2019 14:00

Biology, 20.07.2019 14:00

History, 20.07.2019 14:00

Geography, 20.07.2019 14:00

Mathematics, 20.07.2019 14:00





Computers and Technology, 20.07.2019 14:00

Advanced Placement (AP), 20.07.2019 14:00


History, 20.07.2019 14:00




Mathematics, 20.07.2019 14:00


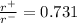 .................1
.................1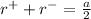 ...................2
...................2