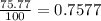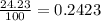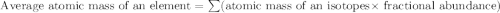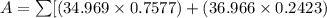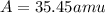Chemistry, 05.09.2019 21:30 anglekhan101
Chlorine has two isotopes, 35cl and 37cl; 75.77% of chlorine is 35cl, and 24.23% is 37cl. the atomic mass of 35cl is 34.969 amu, and the atomic mass of 37cl is 36.966 amu. what is the atomic weight of chlorine?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 23:00
Select the correct answer. given: 2libr + ba → babr2 + 2li in this chemical reaction, 325 grams of barium (ba) react completely. how many moles of lithium (li) are produced? a. 1.18 mol b. 2.37 mol c. 4.73 mol d. 16.4 mol e. 32.9 mol
Answers: 2

Chemistry, 21.06.2019 23:50
2points what is the job of a scientist? a. to answer ethical questions. b. to write laws based on his or her knowledge. c. to ask and answer scientific questions. d. to ignore facts that do not support his or her theory.
Answers: 1

Chemistry, 22.06.2019 08:30
Which common material is an example of a polymer? (25 pts) a. steel b. plastic c. petroleum d. rubbing alcohol
Answers: 2

Chemistry, 22.06.2019 10:50
Someone offer some answers to this, i will give 98 coins and mark as brainliest! i will put the rest of the lab down in the comments,solutions pre-lab questions: in this lab, you will make fruit drinks with powdered drink mix. complete the pre-lab questions to get the values you need for your drink solutions. calculate the molar mass of powered fruit drink mix, made from sucrose (c12h22o11).using stoichiometry, determine the mass of powdered drink mix needed to make a 1.0 m solution of 100 ml. (hint: use molarity = to find the moles of drink mix, then convert moles to grams using a mole conversion.)what mass of powdered drink mix is needed to make a 0.5 m solution of 100 ml?
Answers: 1
You know the right answer?
Chlorine has two isotopes, 35cl and 37cl; 75.77% of chlorine is 35cl, and 24.23% is 37cl. the atomi...
Questions

Mathematics, 29.07.2021 20:20

Mathematics, 29.07.2021 20:20


History, 29.07.2021 20:30





Physics, 29.07.2021 20:30

Social Studies, 29.07.2021 20:30

Computers and Technology, 29.07.2021 20:30


Chemistry, 29.07.2021 20:30

Arts, 29.07.2021 20:30


Chemistry, 29.07.2021 20:30

Mathematics, 29.07.2021 20:30