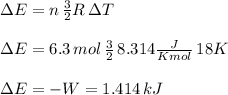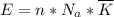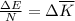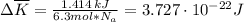Chemistry, 05.09.2019 21:20 jackchelly
The temperature of 6.30 mol of an ideal monatomic gas is raised 18.0 k in an adiabatic process. what are (a) the work w done by the gas, (b) the energy transferred as heat q, (c) the change δeint in internal energy of the gas, and (d) the change δk in the average kinetic energy per atom?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:10
Here’s one way to follow the scientific method. place the missing steps in the correct position in the process
Answers: 1


Chemistry, 22.06.2019 18:00
Which three statements represent the benefits of performing experiments using computer simulations?
Answers: 3

Chemistry, 22.06.2019 19:00
How does a catalyst increase the speed of a reaction? a. the catalyst eliminates the activated complex stage, allowing products to form immediately. b. the catalyst lowers the energy level of the reactants, making it easier for them to react. c. the catalyst makes it easier for the activated complex to form, lowering the activation energy. d. the catalyst raises the energy level of the products, making the reaction finish sooner. reset next
Answers: 1
You know the right answer?
The temperature of 6.30 mol of an ideal monatomic gas is raised 18.0 k in an adiabatic process. what...
Questions



Health, 17.09.2019 08:00



Biology, 17.09.2019 08:00



History, 17.09.2019 08:00



Biology, 17.09.2019 08:00


Mathematics, 17.09.2019 08:00





Mathematics, 17.09.2019 08:00

Biology, 17.09.2019 08:00

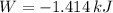 , that means, work is done on the gas, not viceversa.
, that means, work is done on the gas, not viceversa.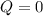 because it is an adiabatic process.
because it is an adiabatic process.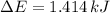
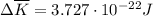 per atom
per atom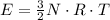 , This result can be experimentally verified or derived from statistical mechanics.
, This result can be experimentally verified or derived from statistical mechanics.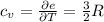
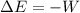
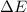 as follows:
as follows: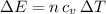 which follows from our first equation.
which follows from our first equation.