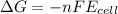Which of the following reactions would be the most spontaneous at 298 k? which of the following reactions would be the most spontaneous at 298 ? a + b → 2c; e∘cell = -0.40 v a + b → c; e∘cell = +1.22 v a + 2b → c; e∘cell = +0.98 v a + b → 3c; e∘cell = +0.15 v more information is needed to determine.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 01:30
(apex) when a cup of water is dropped, as the cup falls, the water in the cup falls out true or false?
Answers: 1

Chemistry, 22.06.2019 02:00
In which of these cases are the two wave points considered to be in phase with each other?
Answers: 1

Chemistry, 22.06.2019 03:00
Match term definition ellipse a) diagonal cross section of a cylinder circle b) diagonal cross section through the widest part of a sphere sphere c) cross section parallel to the base of a cone great circle d) shape created when a semi-circle is rotated around the y-axis triangle e) perpendicular cross section of a cone
Answers: 1

Chemistry, 22.06.2019 11:30
Aperfume bottle is dropped in the corner of a room. the odor of the perfume can be detected on the other side of the room. which statement best describes this observation?
Answers: 2
You know the right answer?
Which of the following reactions would be the most spontaneous at 298 k? which of the following rea...
Questions







Mathematics, 07.09.2021 17:00




Biology, 07.09.2021 17:00

Mathematics, 07.09.2021 17:00

Chemistry, 07.09.2021 17:00


Spanish, 07.09.2021 17:00


English, 07.09.2021 17:00

History, 07.09.2021 17:00

Mathematics, 07.09.2021 17:00