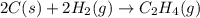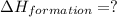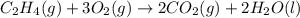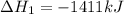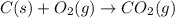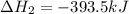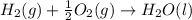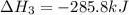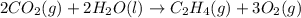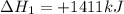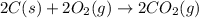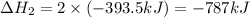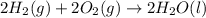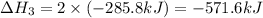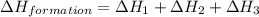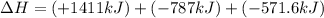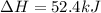Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Often on a topographic map, every fifth contour line is darkened. what is this line called? a. key b.slope c.benchmark d. index contour
Answers: 1

Chemistry, 22.06.2019 01:30
Aroller coaster car is traveling down a track at 22 m/s. the car has a mass of 2000 kg. what is the kinetic energy of the car? a) 22,000 j b) 968,000 j c) 484,000 j d) 44,000 j
Answers: 2


Chemistry, 22.06.2019 12:30
Which element has the lowest electronegativity? calcium(ca) gallium(ga) selenium(se) bromine(br)
Answers: 1
You know the right answer?
Find the standard enthalpy of formation of ethylene, c2h4(g), given the following data: c2h4(g) + 3...
Questions

Chemistry, 03.02.2021 20:20





Biology, 03.02.2021 20:20

Mathematics, 03.02.2021 20:20

Mathematics, 03.02.2021 20:20

Health, 03.02.2021 20:20

Social Studies, 03.02.2021 20:20

Mathematics, 03.02.2021 20:20

Mathematics, 03.02.2021 20:20





Chemistry, 03.02.2021 20:20


Mathematics, 03.02.2021 20:20

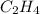 will be,
will be,